As the pandemic caused supply chain organizations and health facilities to temporarily shut down or work at lower capacity all along the public health supply chain, it disrupted the global, national and subnational supply for HIV/AIDS and other public health commodities. It also introduced significant challenges for HIV/AIDS service delivery, including increased risk of COVID-19 exposure for patients and health facility staff.
Understanding the potential adverse impact of COVID-19 on HIV/AIDS services, Ethiopia’s Ministry of Health (MOH) prepared guidelines for provision of HIV/AIDS services in the context of COVID-19, aiming to standardize the national response, reduce the burden on health facilities, reduce non-essential visits of clients and mitigate potential consequences of the pandemic. Per the guidance, the MOH, Ethiopian Pharmaceutical Supply Agency (EPSA) and regional health bureaus (RHBs) established taskforces in May 2020 to ensure continuity of HIV/AIDS services and invited GHSC-PSM to participate.
GHSC-PSM worked with regional taskforces to adapt the interim guidance; address supply chain preparedness and response issues; develop action plans, supervision tools and monitoring tools; and cascade their implementation.
The project continued working to address supply chain challenges of HIV/AIDS commodities through on-site and remote support, conducting rapid assessments on the trend of antiretroviral (ARV) supply dispensed, and facilitating timely resupply, redistribution and stock exchanges. GHSC-PSM supported the transfer of ARVs from health facilities selected for COVID-19 treatment to active HIV/AIDS treatment sites that helped to prevent wastage.
To reduce the risk of contracting COVID-19 at health facilities for clients, the project supported multi-month dispensing (MMD) and provided support on proper counseling and labelling for ARVs to prevent wastage of near-expiry ARVs.
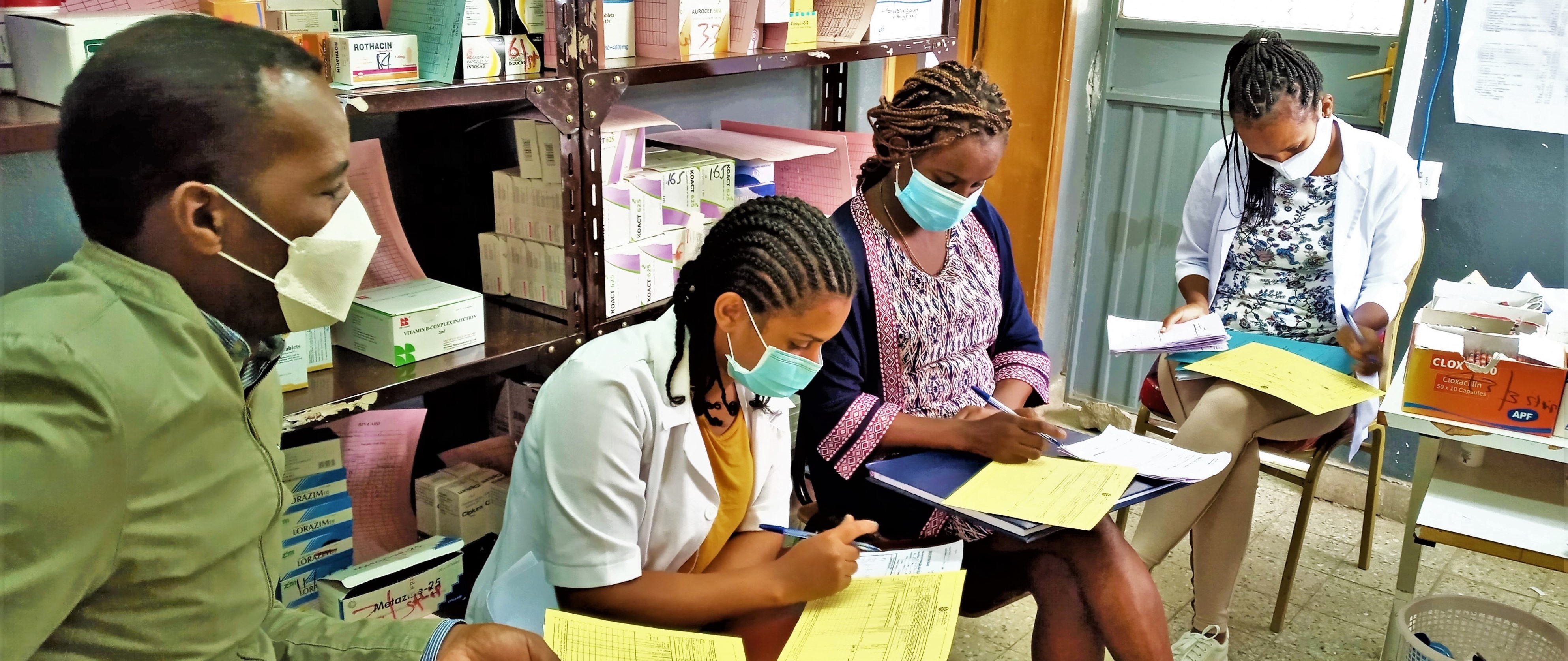
GHSC-PSM also conducted monthly on-site and remote support and stock status monitoring of viral load and early infant diagnosis (VL/EID) reagents, consumables and personal protective equipment at testing sites to monitor the increased demand, as some were used for COVID-19 testing.
In November and December 2020, the project conducted supportive supervision in more than 400 sites on supply chain management of HIV/AIDS commodities, implementation of MMD, stock availability and requesting of ARVs to ensure continuity of HIV services and solve HIV/AIDS commodity management bottlenecks.
GHSC-PSM supported the Ethiopian Pharmaceutical Supply Agency (EPSA) to analyze the biweekly stock status at the national and sub-national levels, procurements and deliveries, and commodity supply pipelines to reduce the supply risks for ARVs, HIV rapid test kits (RTKs) and VL/EID products.
From March to November 2020, EPSA distributed more than 2.8 million packs of Dolutegravir/Lamivudine/Tenofovir and more than 1.5 million packs of Efavirenz/Lamivudine/Tenofovir to support nine months of MMD requirements for 80 percent of HIV/AIDS clients. EPSA also distributed more than 290,000 packs of HIV rapid test kits (RTKs) – enough to test 5.8 million people. In December 2020, inventory analysis of national and subnational level stocks of 24 ARVs and three HIV RTKs indicated that 82 percent of ARVs and all HIV test kits had more than six months stock of supply with no risk category, 14.2 percent had four to six months of supply with low risk category, and 3.6 percent (less frequently used ARVs) had two to four months of supply with medium risk category. As of September 2020, 195,237 adult ART clints on the first line regimens are enrolled on the ASM out of the 308,522 Adults who are eligible for ASM.
These efforts ensured uninterrupted availability of HIV/AIDS commodities and ensured continuity of services, supported acceleration of MMD, alleviated shortages through facilitating redistribution, and minimized expiry and wastage.