GHSC-PSM has developed guidance documents for national and global stakeholders to support global standards implementations in national supply chains.
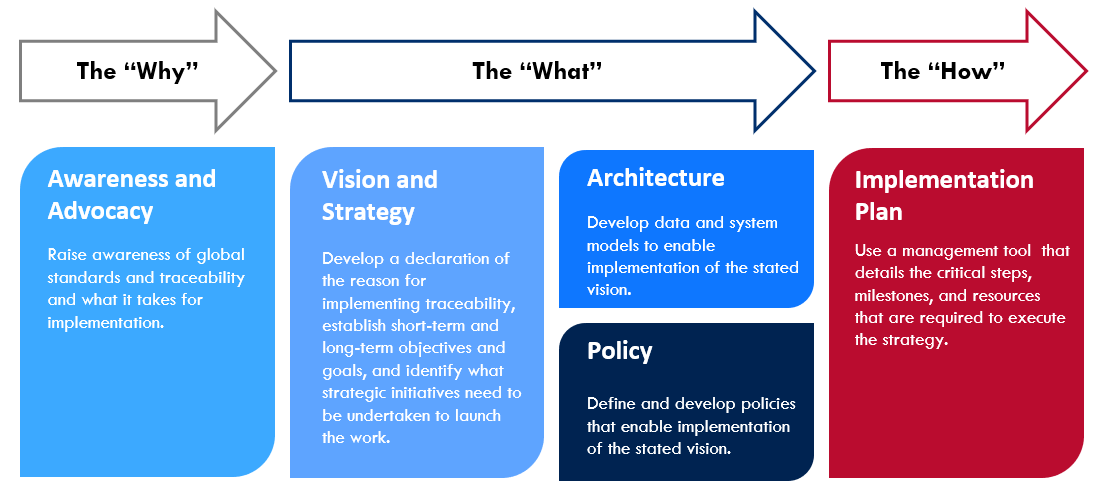
The below process lays out guidance for country programs to systematically organize the work of operationalizing and executing a vision and strategy for pharmaceutical traceability. It serves to orient teams in developing a vision, strategy, and implementation roadmap specific to the country environment – one that considers the key operational components required for successful implementation.
The following are tools comprising the GHSC-PSM Traceability Planning Framework Toolkit*, which cover the 'why,' 'what,' and 'how' to support country programs in implementing global standards. The below toolkit table is fully interactive and any underlined tools can be accessed by clicking on its title:
The "WHY" | The "WHAT" | The "HOW" | ||||||
|---|---|---|---|---|---|---|---|---|
Education & Awareness | Vision & Strategy | Policy & Architecture | Implementation | |||||
Vision | Strategy | Policy | Architecture | Item and Location Identification | Master Data Management | Standards-Based Automatic Identification and Data Capture | ||
Global Standards for Supply Chain Data Visibility Reference Deck | National Pharmaceutical Traceability Vision and Strategy Development Reference Note | Guideline for Identification and Labelling Specification Template and Guidance | ||||||
Traceability Governance Terms of Reference Guidance and Template | Guideline for Pharmaceutical Product & Location Master Data Template and Guidance | Global Standards in LLMICs: Policy Design Considerations to Address Domestic Manufacturer Needs | ||||||
Implementation Guidance for Pharmaceutical Traceability Leveraging GS1 Standards | ||||||||
More information on the implementation of the National Product Catalog as a tool to support MDM can be found on a separate site here.